The Ultimate Guide to Sodium's Valence Electrons
Sodium, a highly reactive alkali metal, holds a significant position in the periodic table. Its unique properties and behavior have intrigued scientists and chemists for centuries. In this comprehensive guide, we will delve into the world of sodium's valence electrons, uncovering their importance, characteristics, and real-world applications.
Understanding Sodium’s Valence Electrons

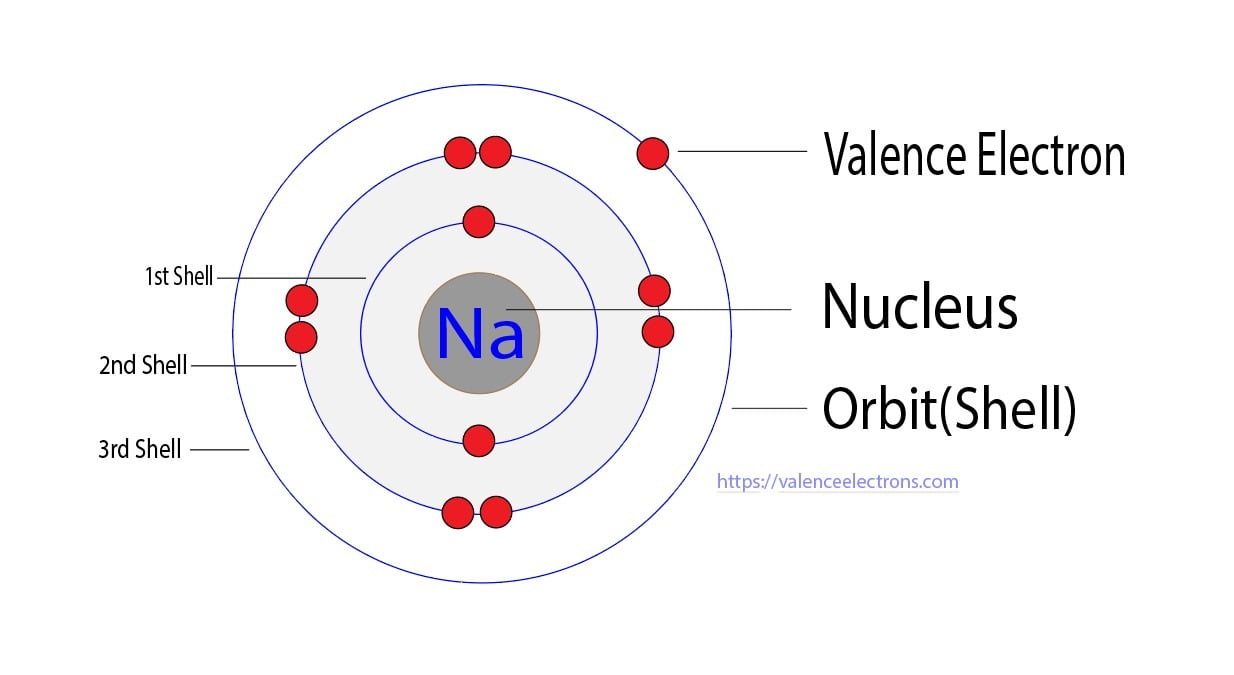
At the heart of sodium’s reactivity lies its valence electrons. Valence electrons are the electrons in the outermost energy level of an atom, also known as the valence shell. These electrons are crucial for an atom’s chemical behavior and bonding capabilities.
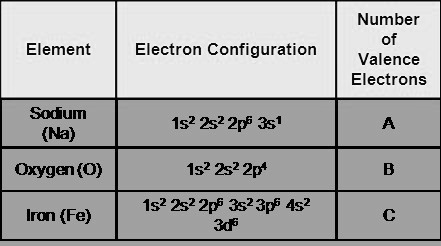
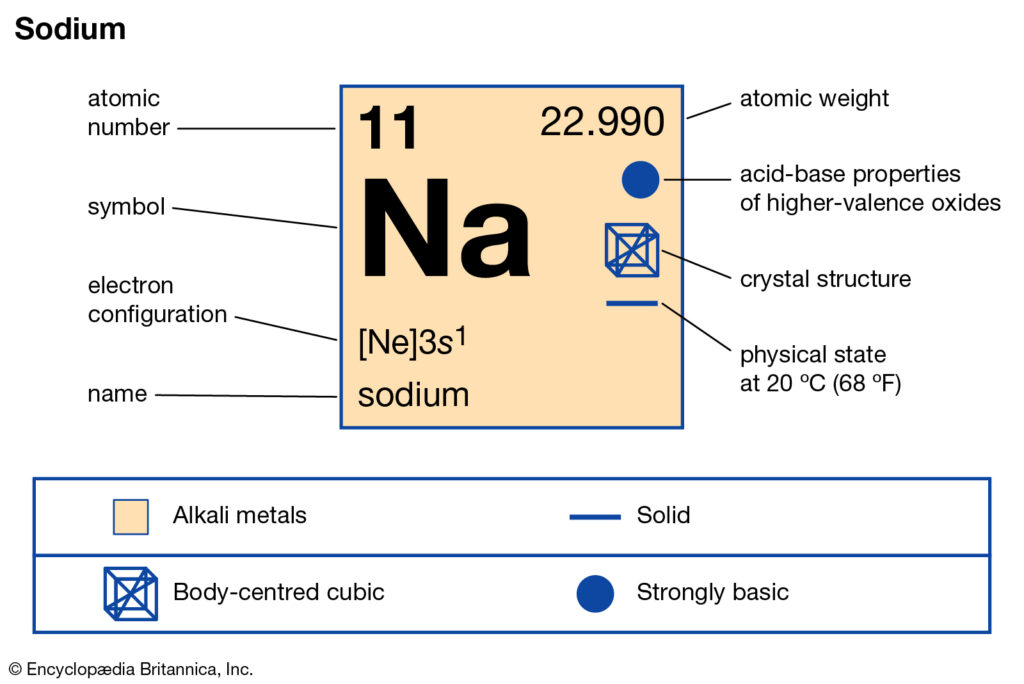
Sodium, with the symbol Na and atomic number 11, belongs to group 1 of the periodic table, the alkali metals. Its electronic configuration is [Ne] 3s1, indicating that its outermost energy level, the 3s orbital, contains a single electron. This lone electron in the valence shell is responsible for sodium's distinctive chemical characteristics.
The Role of Valence Electrons in Sodium’s Reactivity
The presence of a single valence electron makes sodium highly reactive. It eagerly seeks to lose this electron to achieve a stable electronic configuration similar to that of neon, its nearest noble gas neighbor.
When sodium atoms come into contact with other elements, particularly those with a higher electronegativity, they readily donate their valence electron. This electron transfer forms ionic bonds, resulting in the formation of sodium compounds.
For instance, when sodium reacts with chlorine, an extremely electronegative element, it loses its valence electron to chlorine, forming sodium chloride (NaCl), commonly known as table salt. This process demonstrates sodium's valence electrons' crucial role in chemical bonding and the formation of stable compounds.
Sodium’s Valence Electrons in Ionic Compounds
Sodium’s valence electrons play a pivotal role in the formation of various ionic compounds. These compounds are characterized by the complete transfer of electrons from one atom to another, resulting in the formation of ions.
In the case of sodium, its valence electron is donated to other elements, forming positively charged sodium ions (Na+). These sodium ions then combine with negatively charged ions from other elements to create ionic compounds.
| Compound | Formula | Description |
|---|---|---|
| Sodium Chloride (Table Salt) | NaCl | A common ionic compound used in various industries and everyday life. |
| Sodium Hydroxide (Caustic Soda) | NaOH | A strong base widely used in industrial processes and household cleaning products. |
| Sodium Carbonate (Soda Ash) | Na2CO3 | A versatile compound with applications in glass manufacturing, water treatment, and detergents. |
| Sodium Bicarbonate (Baking Soda) | NaHCO3 | A well-known leavening agent in baking and a mild alkaline used for cleaning. |
Real-World Applications of Sodium’s Valence Electrons
The reactivity and electron transfer capabilities of sodium’s valence electrons find extensive applications in various fields, from chemistry and industry to everyday life.
Chemical Industry
Sodium compounds are essential in the chemical industry for diverse purposes. Sodium hydroxide (NaOH), for example, is a highly versatile base used in the production of soaps, detergents, and various chemical processes.
Metal Production
Sodium plays a critical role in metal production, particularly in the extraction of metals from their ores. The highly reactive nature of sodium allows it to reduce certain metal oxides, releasing the metal in its pure form. This process, known as the Sodium Reduction Method, is used in the production of metals like titanium and zirconium.
Energy Storage
Sodium’s valence electrons have also found applications in energy storage systems. Sodium-ion batteries, similar to lithium-ion batteries, offer a promising alternative for energy storage. These batteries utilize the movement of sodium ions during charging and discharging cycles, providing a sustainable and eco-friendly energy solution.
Pharmaceuticals and Healthcare
Sodium compounds are integral to the pharmaceutical industry and healthcare. Sodium chloride (NaCl) is a vital component of intravenous (IV) fluids, ensuring proper hydration and electrolyte balance in patients. Additionally, sodium bicarbonate (NaHCO3) is used in medical treatments to regulate acidity and alkalinity in the body.
Agriculture and Food
In agriculture, sodium compounds are used as fertilizers and soil amendments. Sodium nitrate (NaNO3) and sodium phosphate (Na3PO4) are commonly employed to enhance crop growth and nutrient availability. Furthermore, sodium is present in various food additives, preservatives, and flavor enhancers, making it an essential component of the food industry.
The Future of Sodium’s Valence Electrons

The study of sodium’s valence electrons continues to drive advancements in various fields. Researchers are exploring innovative applications of sodium-based compounds, particularly in sustainable energy solutions and environmental conservation.
The development of sodium-ion batteries, for instance, holds promise for grid-scale energy storage systems, offering a more environmentally friendly and cost-effective alternative to lithium-ion batteries. Additionally, sodium's role in metal extraction and production is being optimized to reduce environmental impact and improve efficiency.
As our understanding of sodium's valence electrons deepens, we can expect further breakthroughs and discoveries, leading to a more sustainable and innovative future.
What is the significance of sodium’s single valence electron?
+Sodium’s single valence electron makes it highly reactive. It readily donates this electron to form ionic bonds with other elements, resulting in the formation of various compounds essential in different industries.
How do sodium’s valence electrons contribute to its reactivity with chlorine to form table salt?
+Sodium’s valence electron is donated to chlorine, an extremely electronegative element. This electron transfer results in the formation of sodium chloride (NaCl), commonly known as table salt, a crucial compound with numerous applications.
What are some real-world applications of sodium compounds beyond the chemical industry?
+Sodium compounds find applications in metal production, energy storage, pharmaceuticals, healthcare, agriculture, and the food industry. From reducing metal oxides to providing essential nutrients in agriculture, sodium’s valence electrons enable a wide range of practical uses.