Essential Tips for Clinical Project Managers
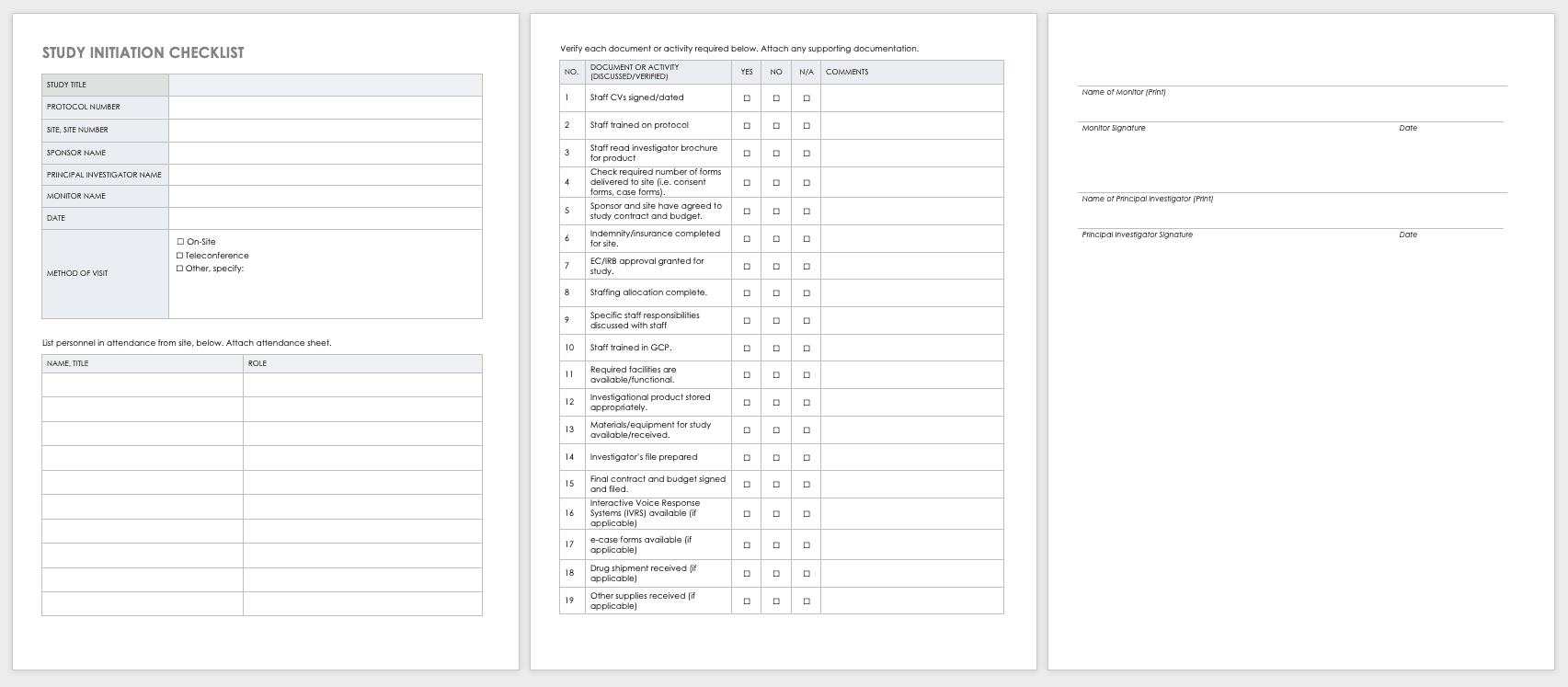
Clinical project management is a complex and multifaceted role that requires a unique set of skills and strategies. For those in the healthcare industry, especially within the realm of clinical research and trials, effective project management is crucial for success. In this comprehensive guide, we will delve into the world of clinical project management, offering essential tips and insights to help professionals navigate this challenging yet rewarding field.
Mastering the Art of Clinical Project Management

Clinical project managers play a pivotal role in the success of medical research and development initiatives. With their expertise and strategic planning, they ensure that clinical trials and projects are executed efficiently, ethically, and within budget. Here, we explore the key aspects of this demanding role and provide practical advice to enhance your project management skills.
1. Understanding the Clinical Research Landscape
The first step to effective clinical project management is a deep understanding of the clinical research process. This involves familiarity with the various stages of a clinical trial, from protocol development to data analysis and reporting. Staying abreast of industry regulations, such as Good Clinical Practice (GCP) guidelines, is also crucial for ensuring ethical and compliant practices.
| Clinical Trial Stages | Key Considerations |
|---|---|
| Protocol Design | Involves defining the research question, study design, and ethical considerations. |
| Patient Recruitment | Strategies to attract and retain suitable participants, while maintaining confidentiality. |
| Data Collection & Management | Ensuring accurate, timely data capture and secure storage. |
| Analysis & Reporting | Interpreting results and presenting them clearly to stakeholders. |

Moreover, clinical project managers must be adept at navigating the unique challenges posed by different therapeutic areas. Whether it's managing rare disease studies or large-scale multi-center trials, each project brings its own set of complexities.
2. Building a Collaborative Team
Clinical research is a team effort, involving collaboration between various stakeholders. As a project manager, one of your primary responsibilities is to foster an environment of collaboration and open communication. This includes effectively managing relationships with investigators, research sites, sponsors, and regulatory bodies.
- Investigator Engagement: Building strong relationships with principal investigators ensures buy-in and commitment to the project's success.
- Site Management: Regular communication and support to research sites can help overcome challenges and ensure timely progress.
- Sponsor Communication: Clear and frequent updates to sponsors are essential for maintaining funding and alignment with project goals.
Additionally, project managers should focus on continuous professional development for their team members. This may involve providing training opportunities, mentorship programs, or access to industry conferences and workshops.
3. Risk Assessment and Mitigation
Clinical trials are inherently risky endeavors, with potential challenges ranging from recruitment delays to data integrity issues. Effective project managers must be adept at identifying, assessing, and mitigating these risks.
One key strategy is to conduct a thorough risk assessment at the project's onset. This involves identifying potential threats to the project's timeline, budget, or objectives, and developing contingency plans to address them. Regular risk reviews throughout the trial can help ensure that any emerging issues are addressed promptly.
| Common Clinical Trial Risks | Mitigation Strategies |
|---|---|
| Patient Recruitment Delays | Implement targeted recruitment strategies, such as social media campaigns or patient registries. |
| Data Quality Concerns | Establish robust data management protocols and conduct regular quality assurance checks. |
| Regulatory Compliance Issues | Stay informed about evolving regulations and ensure all team members are trained on compliance requirements. |
4. Effective Communication and Documentation
Clear and concise communication is essential in clinical project management. This involves regular updates to stakeholders, as well as detailed documentation of project activities and decisions.
- Status Reports: Provide timely updates on project progress, highlighting key achievements and upcoming milestones.
- Meeting Minutes: Document important discussions and decisions from team meetings to ensure transparency and accountability.
- Issue Logs: Maintain a centralized record of issues, risks, and action items to ensure they are addressed and resolved.
Furthermore, clinical project managers should be skilled in adapting their communication style to suit different audiences. Whether it's explaining complex research concepts to laypersons or presenting data to senior executives, effective communication is key to project success.
5. Utilizing Technology and Data Analytics
In today’s digital age, technology and data analytics play a crucial role in clinical project management. From electronic data capture systems to advanced analytics tools, these innovations can streamline processes and enhance decision-making.
- Electronic Data Capture (EDC): Utilize EDC systems to improve data quality and reduce manual errors.
- Data Analytics: Leverage analytics tools to identify trends, forecast outcomes, and optimize trial design.
- Project Management Software: Adopt robust software solutions to manage timelines, budgets, and resource allocation.
However, with the increasing reliance on technology comes the need for robust cybersecurity measures. Clinical project managers should ensure that all data is securely stored and protected against potential breaches.
6. Continuous Learning and Adaptation
The field of clinical research is ever-evolving, with new therapies, technologies, and regulations constantly emerging. As such, clinical project managers must embrace a culture of continuous learning and adaptation.
This involves staying abreast of industry developments, attending relevant conferences and workshops, and engaging in ongoing professional development. By staying informed and adaptable, project managers can ensure that their skills remain relevant and effective in a rapidly changing landscape.
Conclusion

Clinical project management is a challenging but highly rewarding career path. By mastering the art of clinical research, building collaborative teams, and staying agile in the face of challenges, project managers can ensure the success of their initiatives. With the right strategies and a commitment to continuous learning, the possibilities for growth and impact in this field are limitless.
How can I stay up-to-date with industry regulations and best practices in clinical project management?
+Attending industry conferences, webinars, and workshops can provide valuable insights into the latest regulations and best practices. Additionally, subscribing to reputable industry publications and joining relevant professional associations can help you stay informed.
What are some common challenges faced by clinical project managers, and how can they be overcome?
+Common challenges include recruitment delays, data integrity issues, and budget constraints. To overcome these, project managers can implement targeted recruitment strategies, establish robust data management protocols, and engage in proactive budget management and resource allocation.
How can clinical project managers ensure effective communication with diverse stakeholders?
+Clinical project managers should tailor their communication style to suit the needs and expertise of each stakeholder. This may involve using lay language for non-expert audiences and providing detailed, technical explanations for more specialized stakeholders. Regular feedback and open communication channels can also help ensure that everyone is on the same page.



