How Much is 100mg in ml?
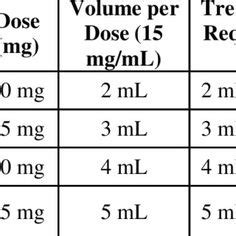
When converting between milligrams (mg) and milliliters (ml), it's important to note that this conversion is specific to certain substances and their densities. The conversion factor can vary greatly depending on the substance in question, as different substances have different densities.
In this article, we will delve into the process of converting 100 milligrams to milliliters, exploring the factors that influence this conversion and providing real-world examples to illustrate the concept.
Understanding the Conversion: Milligrams to Milliliters

The conversion from milligrams to milliliters is a common calculation in various fields, including medicine, chemistry, and nutrition. It is a crucial step in understanding the dosage or concentration of a substance, especially when dealing with precise measurements.
The fundamental relationship between milligrams and milliliters is governed by the concept of density. Density, often represented as the symbol ρ, is a measure of the mass of a substance per unit volume. It is typically expressed in units such as grams per milliliter (g/ml) or kilograms per liter (kg/L). The density of a substance determines how much of it can be packed into a given volume.
To convert milligrams to milliliters, we need to know the density of the specific substance in question. The formula for this conversion is as follows:
Volume (ml) = Mass (mg) / Density (mg/ml)
This formula essentially divides the mass of the substance by its density to determine the corresponding volume.
Example 1: Water
Let's consider a straightforward example using water, which has a well-known density of 1 gram per milliliter (1 g/ml) at standard temperature and pressure. If we have 100 milligrams of water, the conversion to milliliters would be:
Volume (ml) = 100 mg / 1 g/ml
By performing this calculation, we find that 100 milligrams of water is equivalent to 0.1 milliliters (or 0.1 ml). This is a simple and accurate conversion due to water's consistent density.
Example 2: Pharmaceutical Solutions
In the pharmaceutical industry, accurate dosage calculations are vital. Let's take the example of a medication with a density of 1.2 grams per milliliter (1.2 g/ml). If a patient needs to take a dose of 100 milligrams of this medication, the conversion to milliliters would be:
Volume (ml) = 100 mg / 1.2 g/ml
Solving this equation, we find that 100 milligrams of this medication correspond to approximately 0.0833 milliliters (or 83.3 μl). This precise calculation ensures the patient receives the correct dosage.
Example 3: Concentrated Chemicals
When dealing with concentrated chemicals, the density can vary significantly. For instance, consider a highly concentrated acid with a density of 1.5 grams per milliliter (1.5 g/ml). If a chemist needs to measure out 100 milligrams of this acid, the conversion would be:
Volume (ml) = 100 mg / 1.5 g/ml
In this case, 100 milligrams of the acid are equivalent to approximately 0.0667 milliliters (or 66.7 μl). Accurate conversions like this are essential to ensure safety and effectiveness in chemical experiments.
Factors Affecting the Conversion

As illustrated in the examples above, the key factor influencing the conversion from milligrams to milliliters is the density of the substance. Density can vary significantly depending on the substance's composition, temperature, and pressure.
Here are some key factors to consider:
- Substance Type: Different substances have unique densities. For example, the density of water is constant, but the density of a pharmaceutical solution or a chemical compound can vary widely.
- Temperature and Pressure: The density of a substance can change with temperature and pressure. This is particularly important for gases and certain liquids, as their densities can fluctuate significantly.
- Precision and Accuracy: When dealing with precise measurements, it's crucial to use accurate density values. Even small variations in density can lead to significant differences in the converted volume.
Practical Applications
The conversion from milligrams to milliliters has a wide range of practical applications across various industries and fields:
- Pharmaceuticals: Accurate dosage calculations are essential in the pharmaceutical industry to ensure patient safety and effectiveness of medications.
- Chemistry and Laboratory Work: Chemists and researchers rely on precise conversions to ensure the accuracy of chemical reactions and experiments.
- Nutrition and Dietetics: In nutrition, conversions between milligrams and milliliters are used to determine the concentration of nutrients in foods and supplements.
- Manufacturing and Engineering: Engineers and manufacturers often need to convert between mass and volume when designing and producing products.
Common Misconceptions
It's important to address some common misconceptions surrounding the conversion from milligrams to milliliters:
- One-to-One Conversion: Contrary to some beliefs, milligrams and milliliters are not directly interchangeable. The conversion factor depends on the density of the substance, and this relationship is not constant for all substances.
- Volume vs. Weight: Volume (milliliters) and weight (milligrams) are different physical properties. Converting between them requires knowledge of the substance's density, as mass and volume are not equivalent measures.
Safety Considerations

When dealing with substances, especially in medical or chemical contexts, it's crucial to prioritize safety. Here are some key safety considerations:
- Accuracy: Always use accurate and up-to-date density values to ensure precise conversions. Inaccurate conversions can lead to dosage errors or safety hazards.
- Labeling and Documentation: Clearly label substances with their densities and ensure that documentation, such as prescription labels or chemical storage information, is accurate and easily accessible.
- Professional Guidance: For medical or chemical applications, consult with professionals who are trained in handling and measuring substances. They can provide expert guidance and ensure safe practices.
Future Implications and Innovations
As technology advances, the process of converting between milligrams and milliliters is becoming increasingly precise and automated. Here are some future implications and potential innovations:
- Smart Dosage Systems: Developing smart dosage systems that automatically calculate and dispense precise dosages based on weight and density could enhance safety and accuracy in medical settings.
- Advanced Density Sensors: Innovative density sensors could provide real-time density measurements, making it easier to convert between mass and volume accurately.
- AI-Assisted Conversions: Artificial intelligence algorithms can assist in predicting density values for various substances, improving the accuracy of conversions.
The conversion between milligrams and milliliters is a fundamental concept with wide-ranging applications. By understanding the role of density and applying accurate conversions, we can ensure precise measurements and safe practices across various industries. As technology advances, we can expect further innovations to enhance the accuracy and safety of these conversions.
How do I find the density of a substance for accurate conversions?
+You can find the density of a substance through various sources, such as scientific databases, material safety data sheets (MSDS), or by conducting density measurements yourself. Online resources and reference materials often provide reliable density values for common substances.
Can I use a conversion factor instead of calculating the volume directly?
+While it may be tempting to use a conversion factor, it’s important to remember that the conversion factor depends on the substance’s density. Using a generic conversion factor without considering density can lead to inaccurate results. Always calculate the volume based on the specific density of the substance.
What if the density of the substance changes with temperature or pressure?
+If the density of a substance is temperature- or pressure-dependent, it’s crucial to use the density value that corresponds to the specific conditions you’re working with. Ensure that the temperature and pressure are accurately measured and accounted for when performing the conversion.



